Welcome To Visit Us
- Arab Health, Dubai, UAE, January27-29, 2025
- DGNC, Hanover, Germany, June1-4, 2025
- FIME, Miami, USA, June11-13, 2025
- EANS, Vienna, Austria,October5-9, 2025
- MEDICA, Düsseldorf, Germany, November 17-20, 2025
- WFNS, Dubai, UAE, December1-4, 2025

CE Certified!
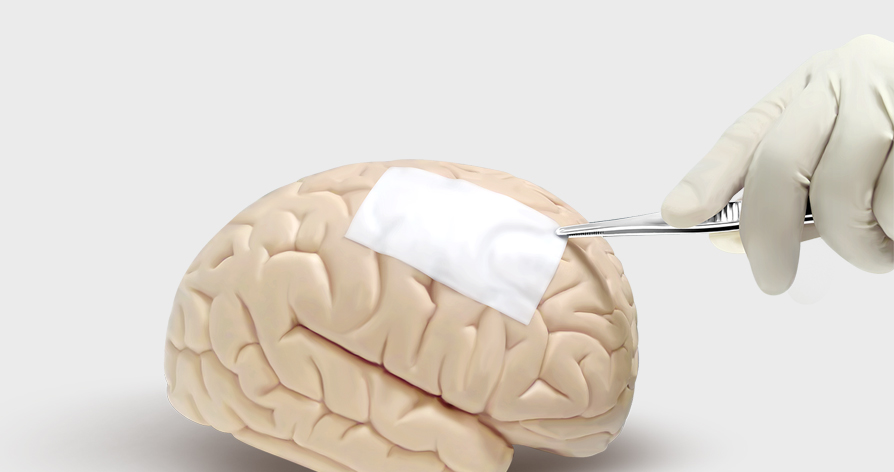
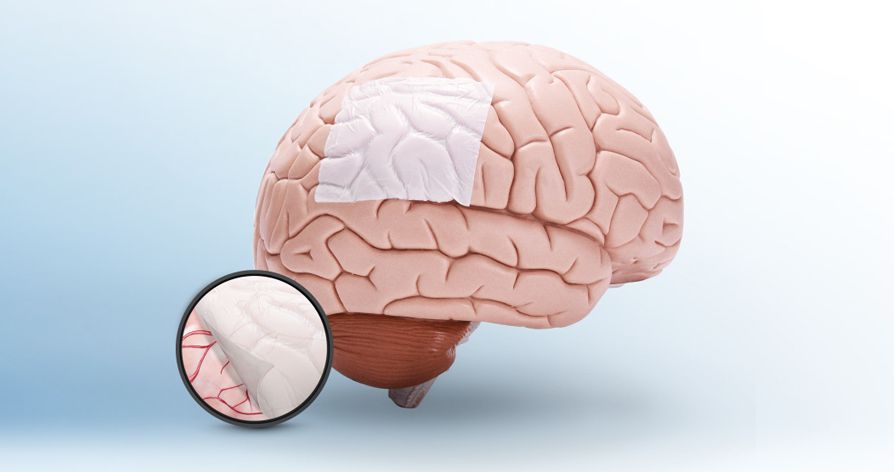
StypCel™ Absorbable Hemostat
StypCel™ Absorbable Hemostat
Your health is all that matters.

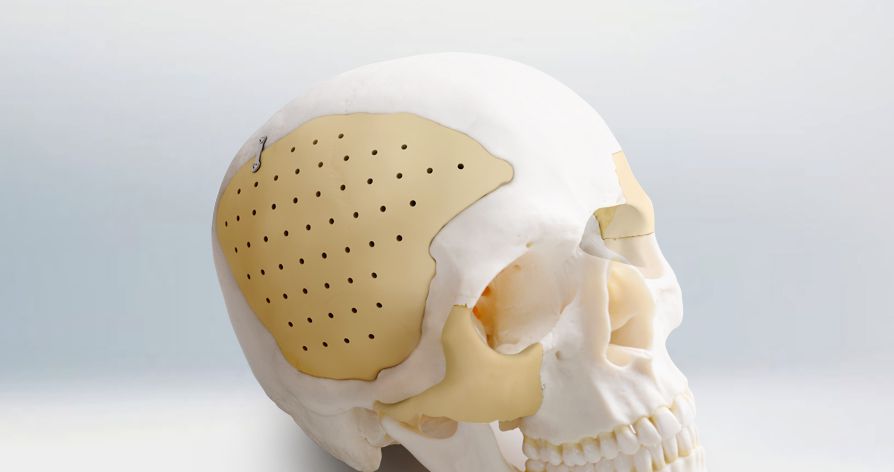
New trend of cranioplasty
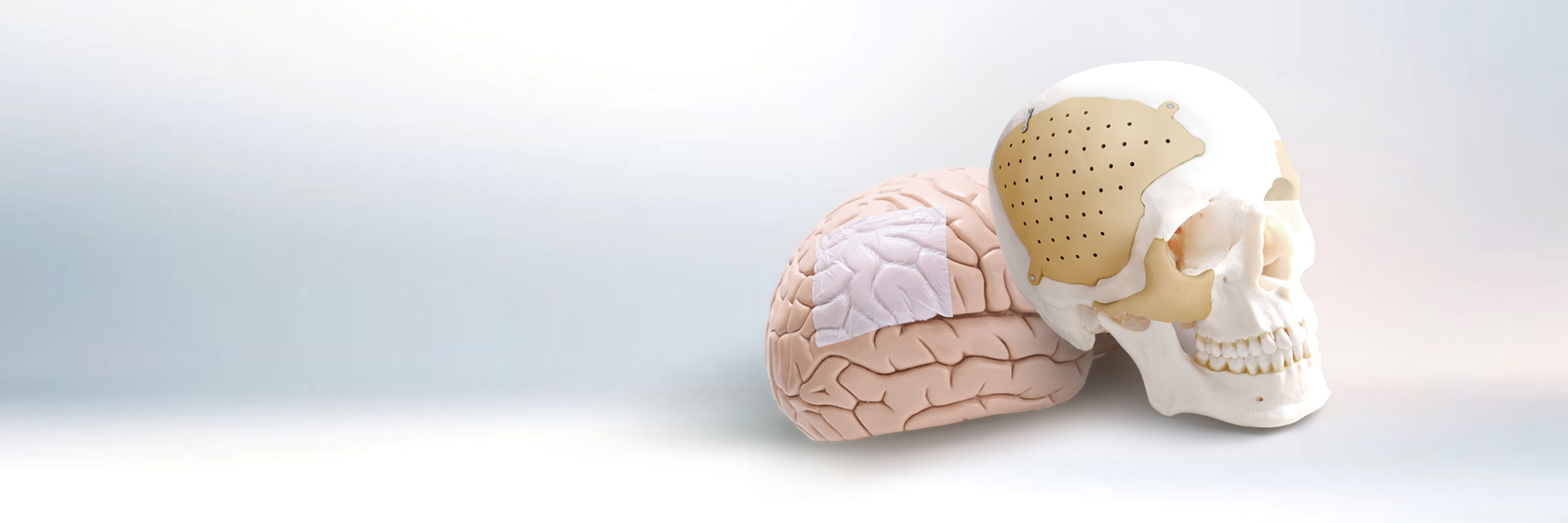
Neurosurgical Solution Provider
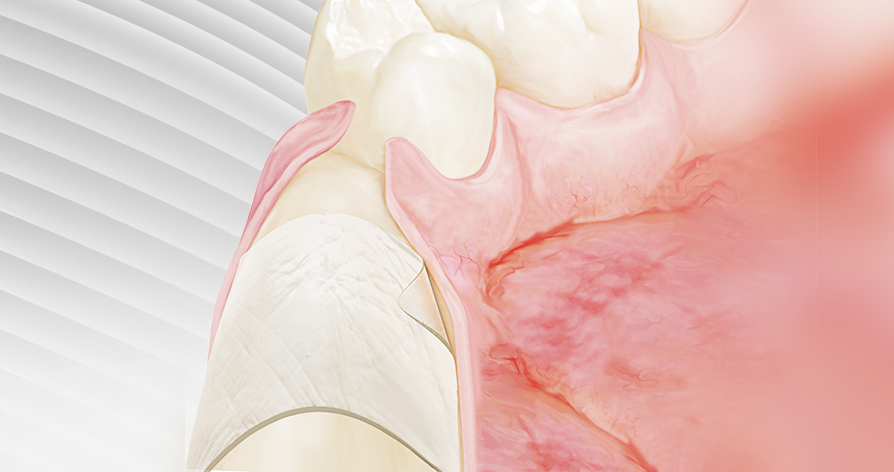
ReDura™, NeoDura™, Recranio™
- Cranial Closure