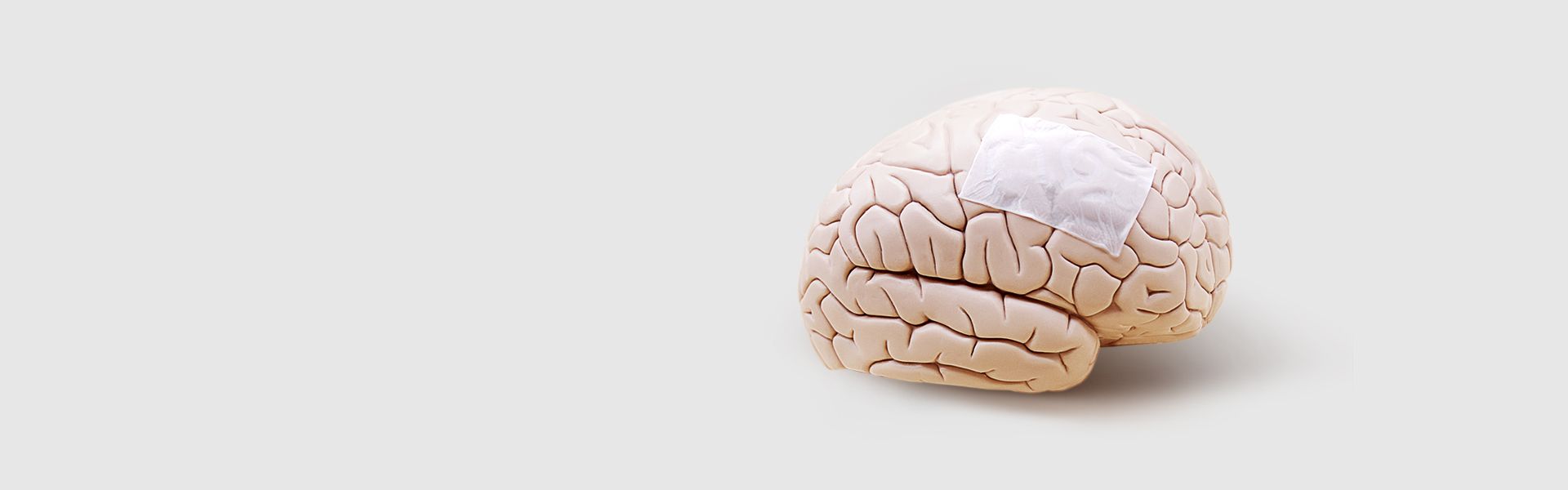
ReDura™ Synthetic Dural Substitute
FDA approved degradable material poly-L-lactic acid (PLA) Widely applied in 60+ countries. Outstanding efficacy and safety for the dural defect repair
Biomimetic
Similar to native extracellular matrix (ECM), rapid repair and regeneration.
Patent Technology
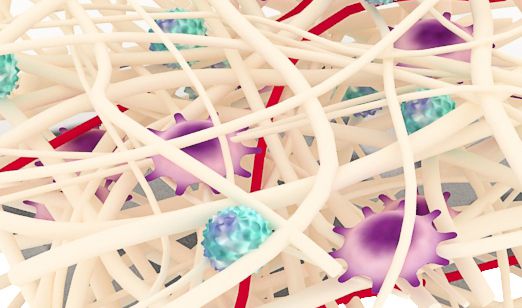
ReDura™ Mimic ECM Structure
Non-animal materials
Biomimetic-Synthetic-Absorbable Dural Substitute without increased risk of animal infection
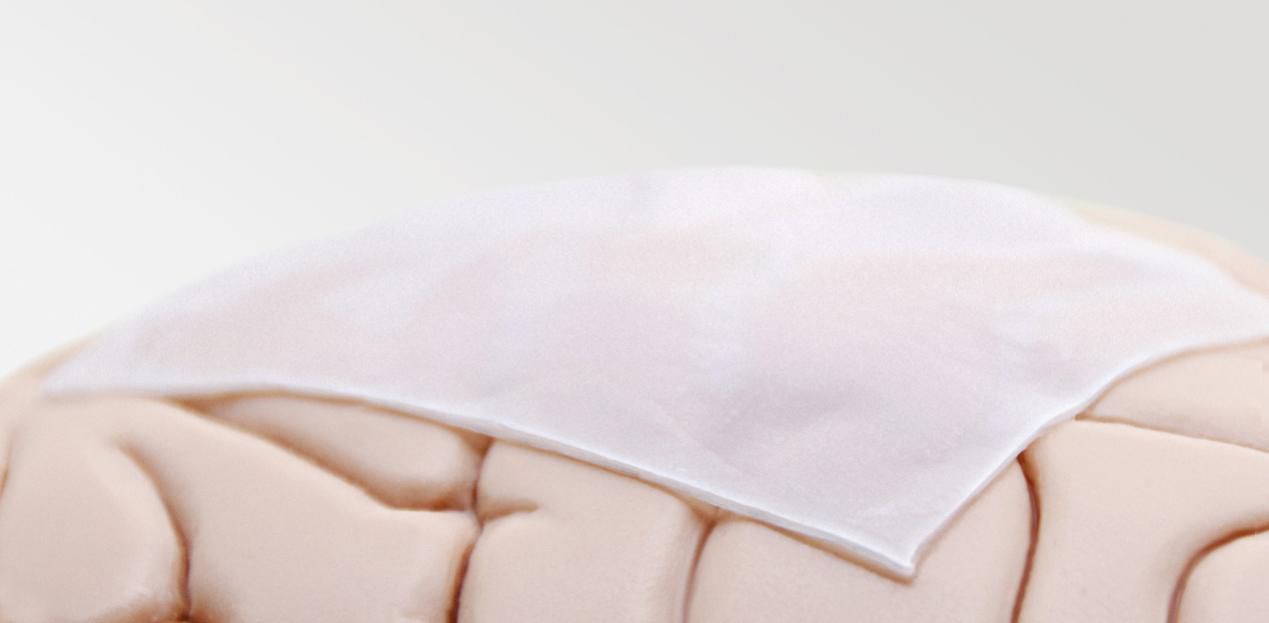
Onlay or Suture
Depends on surgeon habit with flexibility
Onlay
Suture
IFU
LABEL
Document Name
Status
Publication Date
You may have an access to the SSCP by sending an email to information@medprin.com, before the SSCP module of EUDAMED is functional.
