Welcome To Visit Us
- Arab Health, Dubai, UAE, Feb 09-12, 2026
- HOSPITALAR, St.Paul, Brazil, May 19-22, 2026
- DGNC, Aachen, Germany, June 07-10, 2026
- FIME, Miami, USA, June 17-19, 2026
- EANS, Hamburg, Germany, Sep 30 - Oct 04, 2026
- MEDICA, Düsseldorf, Germany, Nov 16-19, 2026

CE Certified!
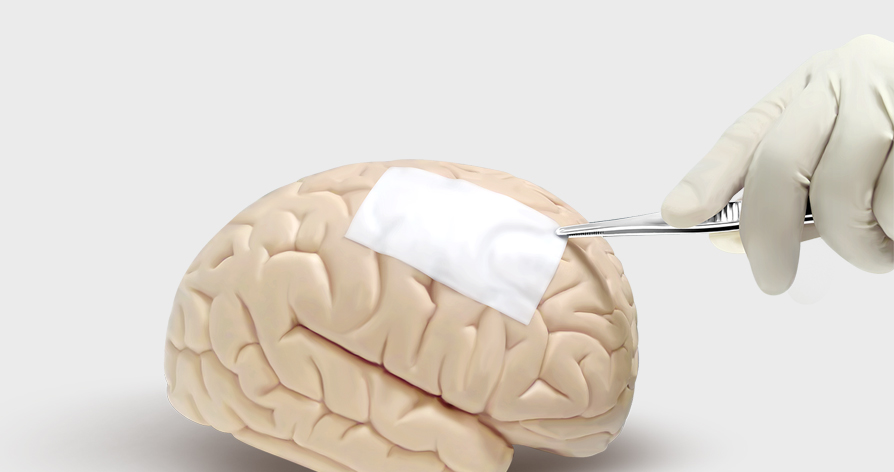
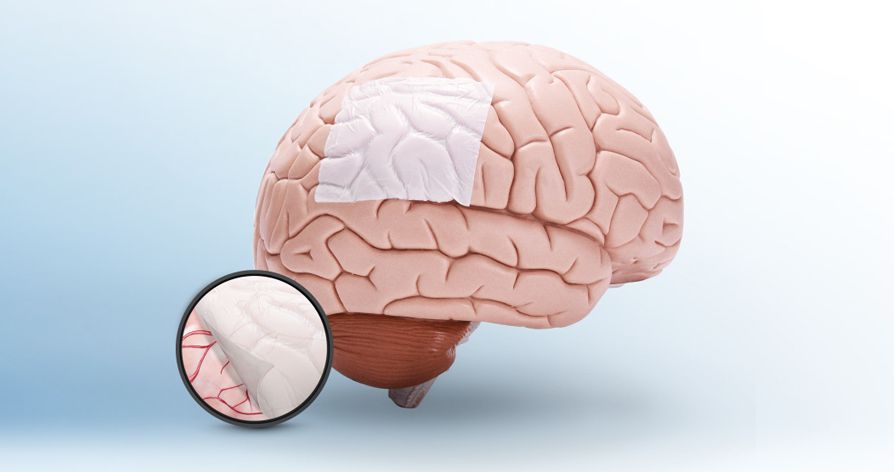
StypCel™ Absorbable Hemostat
StypCel™ Absorbable Hemostat
Your health is all that matters.

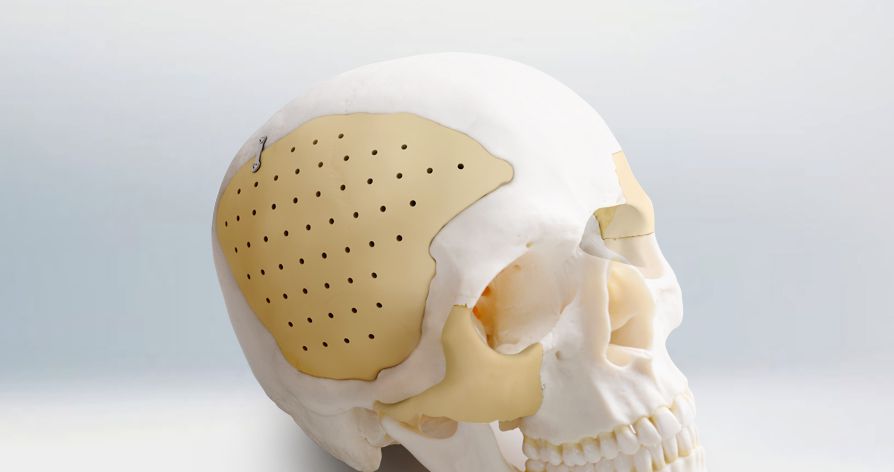
New trend of cranioplasty
Neurosurgical Solution Provider
ReDura™, NeoDura™, Recranio™
- Cranial Closure
MEDPRIN News

WHX Dubai 2026 | Expanded Integrated Solutions, Refreshed Global Brand
2026.02.10

Case Report|Surgical Resection of Brainstem Cavernous Malformati
2025.12.03

Skull Base and Sellar Reconstruction & CSF Leak Control
2025.11.19
The Pituitary Frontier Webinar (5)

Intraoperative Technical Highlights: T-holding “Chopstick” Endoscopic Technique
2025.11.19
The Pituitary Frontier Webinar (4)

Endonasal decompression plus transcranial for a giant sellar–suprasellar tumor case
2025.11.19
The Pituitary Frontier Webinar (3)

Surgery planning w/o MRI, functioning pituitary adenomas involving the cavernous sinus
2025.11.19
The Pituitary Frontier Webinar (2)

Intraoperative lumbar drainage in large pituitary macroadenoma
2025.11.14
The Pituitary Frontier Webinar(1)

SBNS 2025 Briefing Part 2 - Neodura™ in Paediatric duroplasty
2025.10.13

SBNS 2025 Briefing Part 1 - Redura™ in the UK —Ten Years Key Insights
2025.09.29

Congratulations to our partners in Russia for the successful congress
2021.06.25
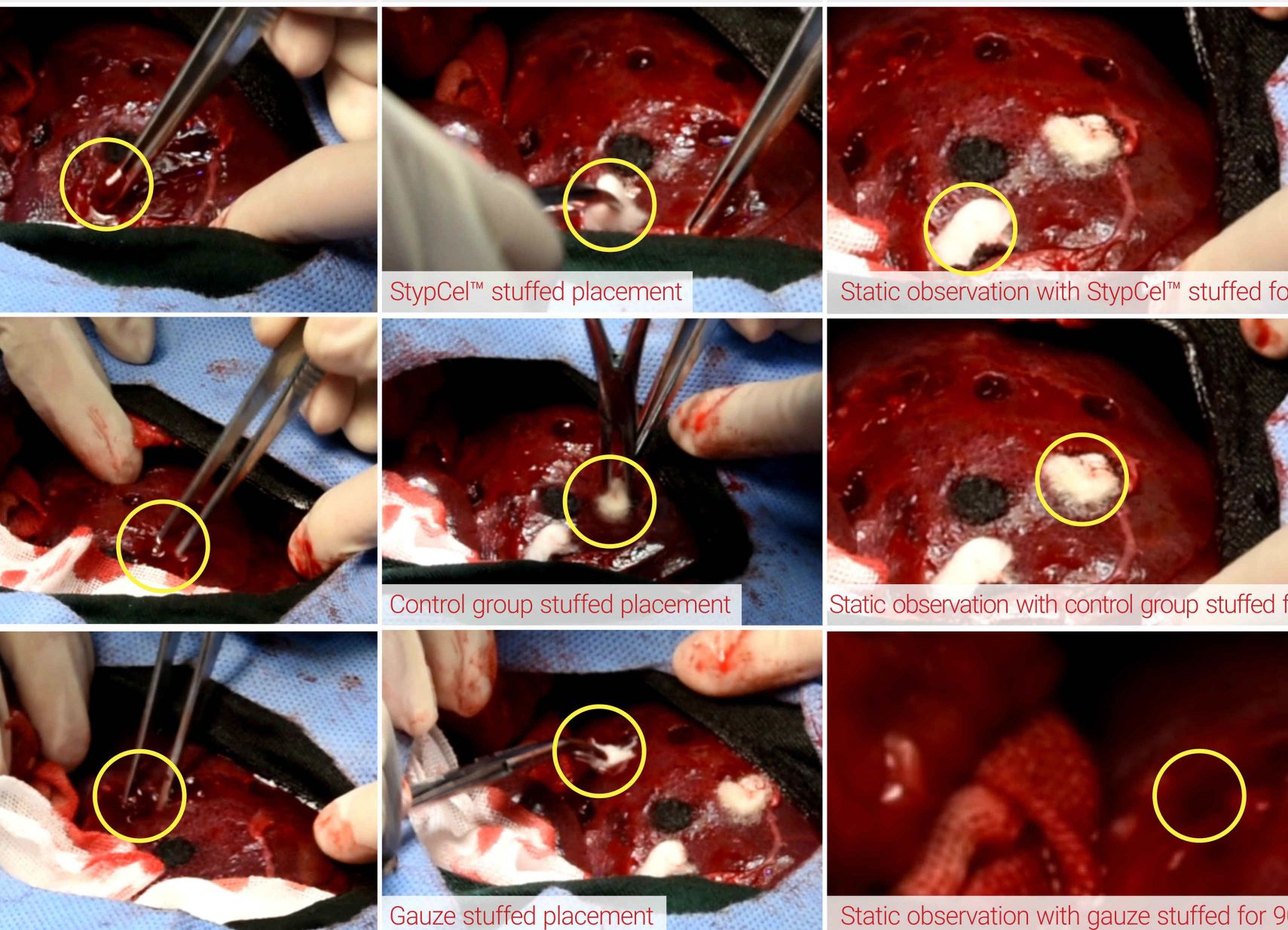
Pre-clinical experiment with swine model
2021.05.08